Merino Trial
Manuel merino former speaker of congress had been in the post less than a week.

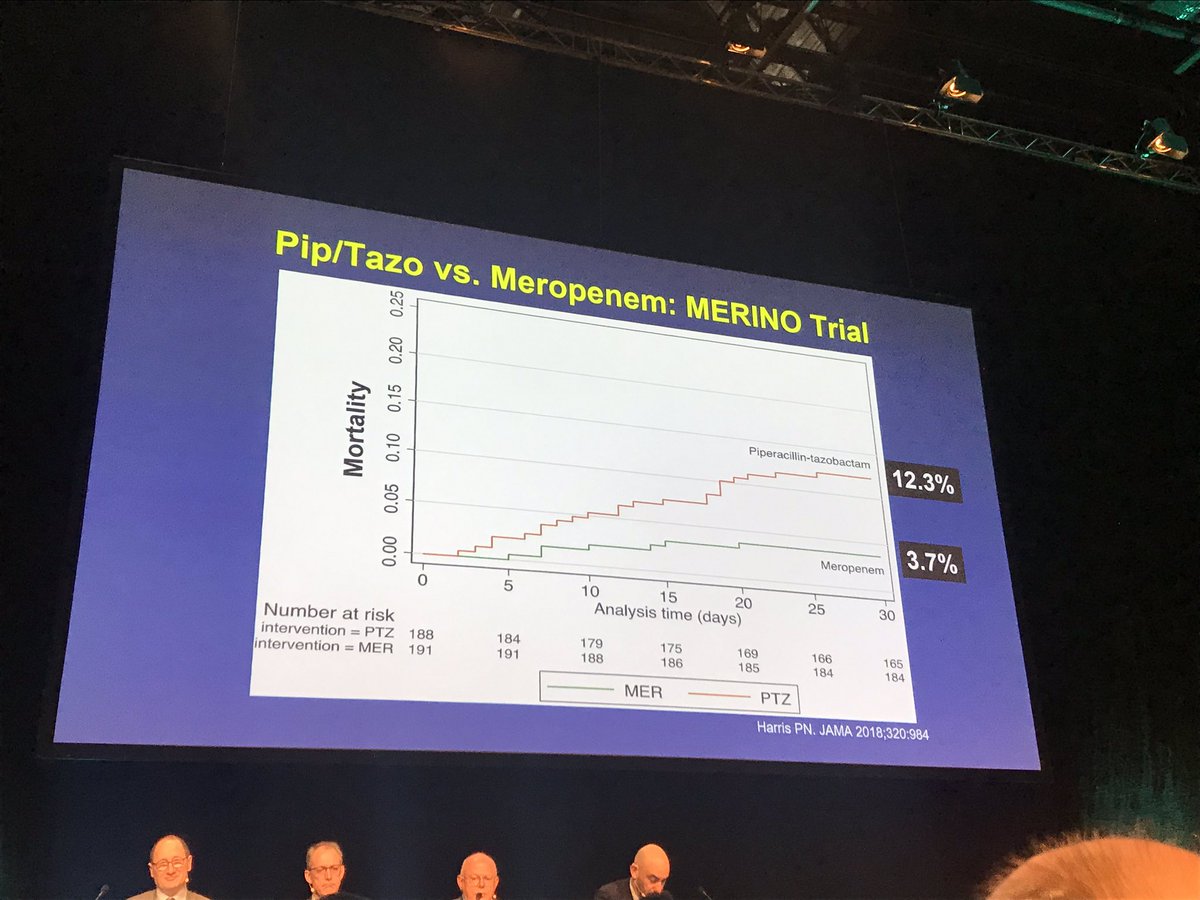
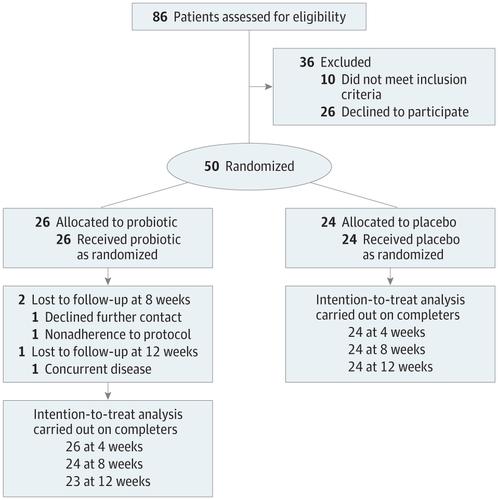
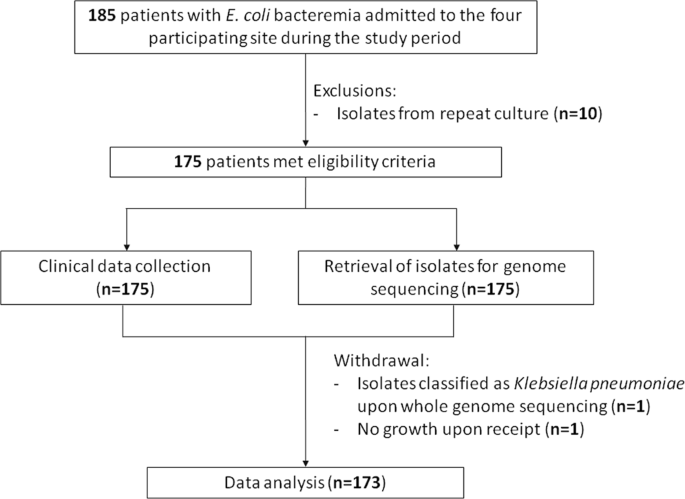
Merino trial. Manuel merino was sworn in as the new peruvian president tuesday after perus congress ousted president martin vizcarra a day earlier in an impeachment vote over corruption allegations prompting. The merino trial is the first randomized controlled trial to evaluate piperacillintazobactam versus meropenem for the definitive treatment of bsis caused by ceftriaxone not susceptible e coli or k pneumoniae that remained susceptible to piperacillintazobactam by current breakpoints. Perus interim president manuel merino is resigning just six days after the impeachment of his predecessor plunged the south american nation into turmoil.
He replaced president martin vizcarra who last monday was removed in an impeachment procedure over bribery. At this time when the country is. Merino a member of the center right popular action party who had been the head of congress moved quickly to swear in a new cabinet this week after vizcarra was removed on monday.
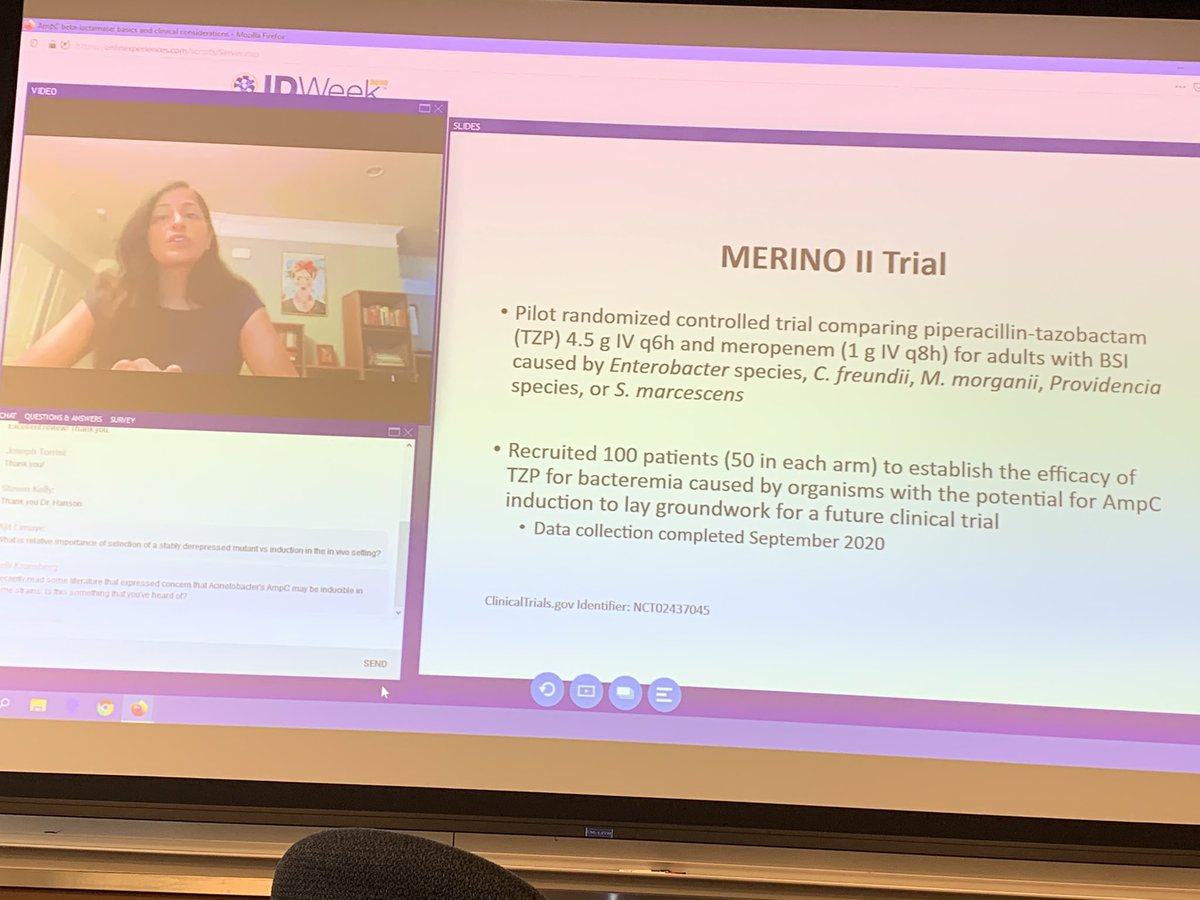
The trial was designed by a steering committee comprising members of the german breast group the national surgical adjuvant breast and bowel project nsabp foundation independent investigators. Coli or klebsiella pneumoniae. The merino trial offered to overcome some of the discrepancies seen in the previous studies.
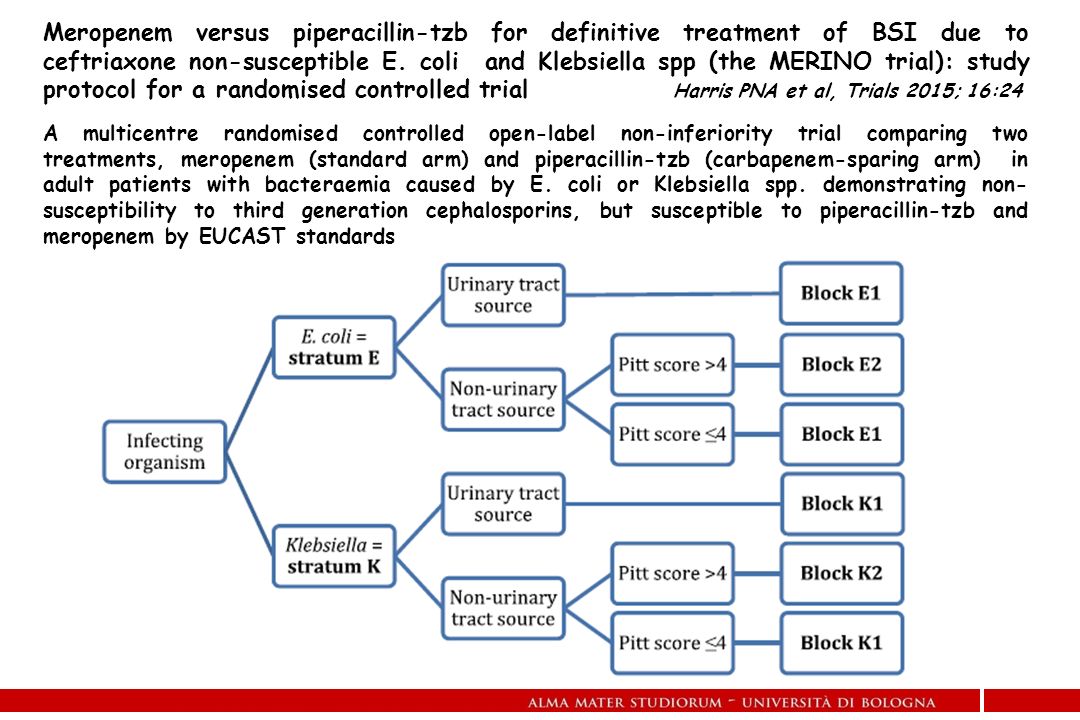
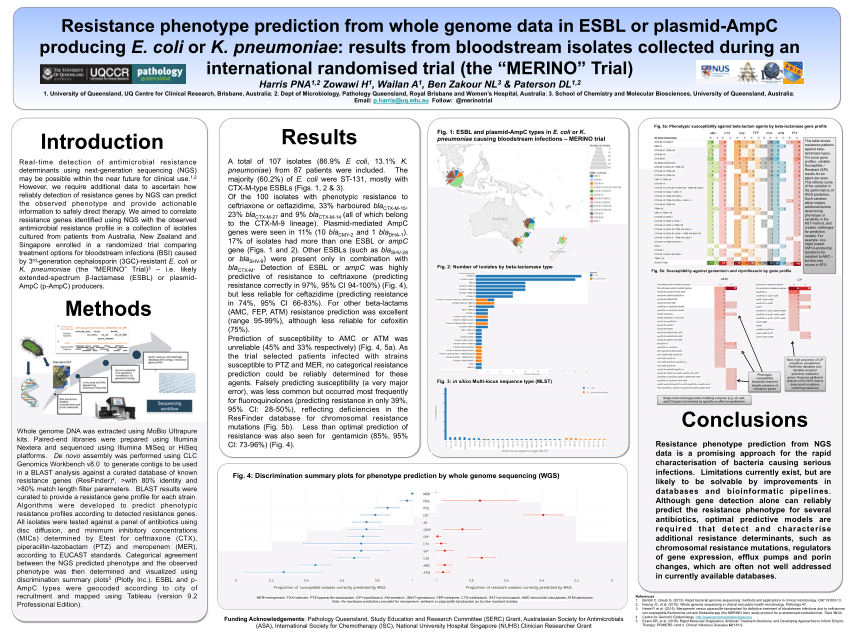
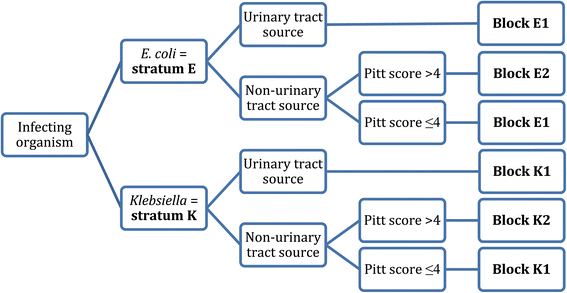
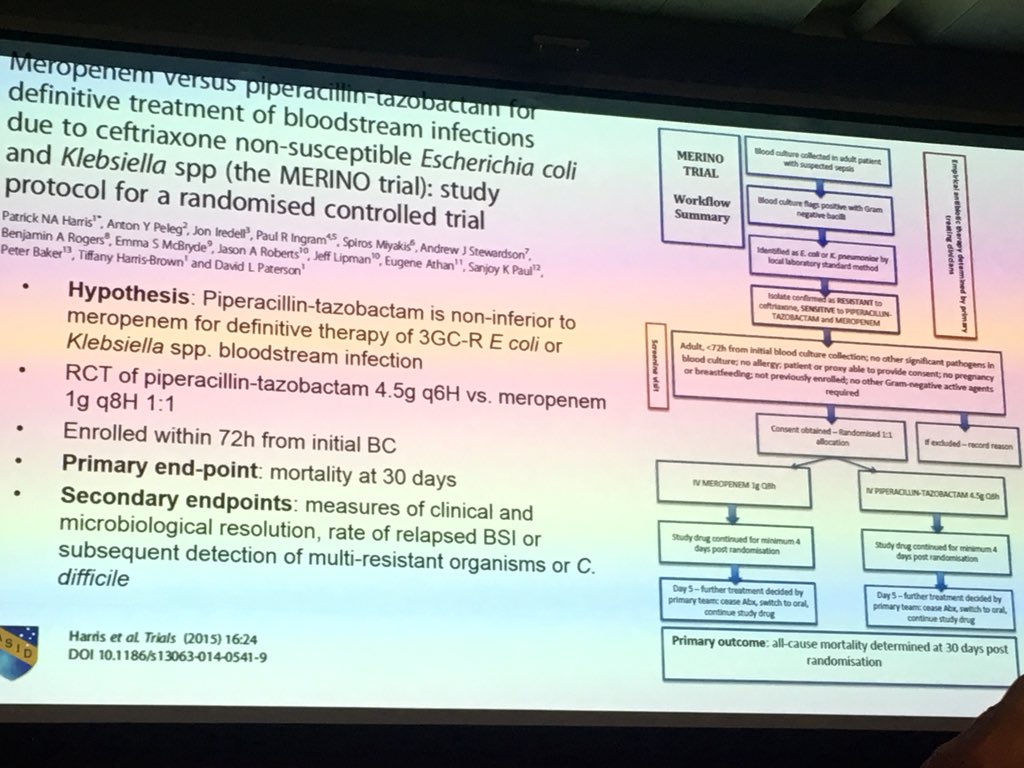
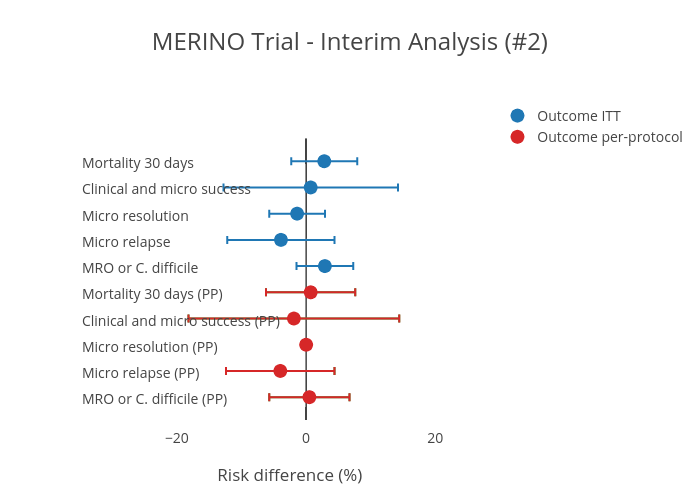
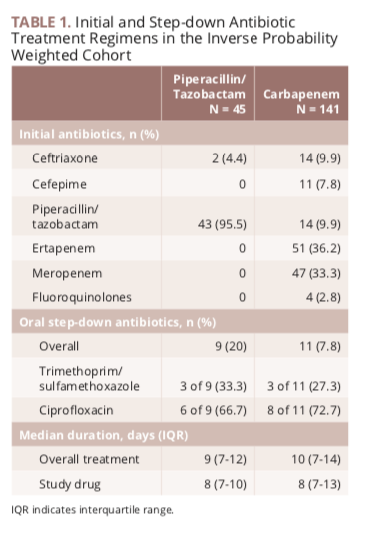
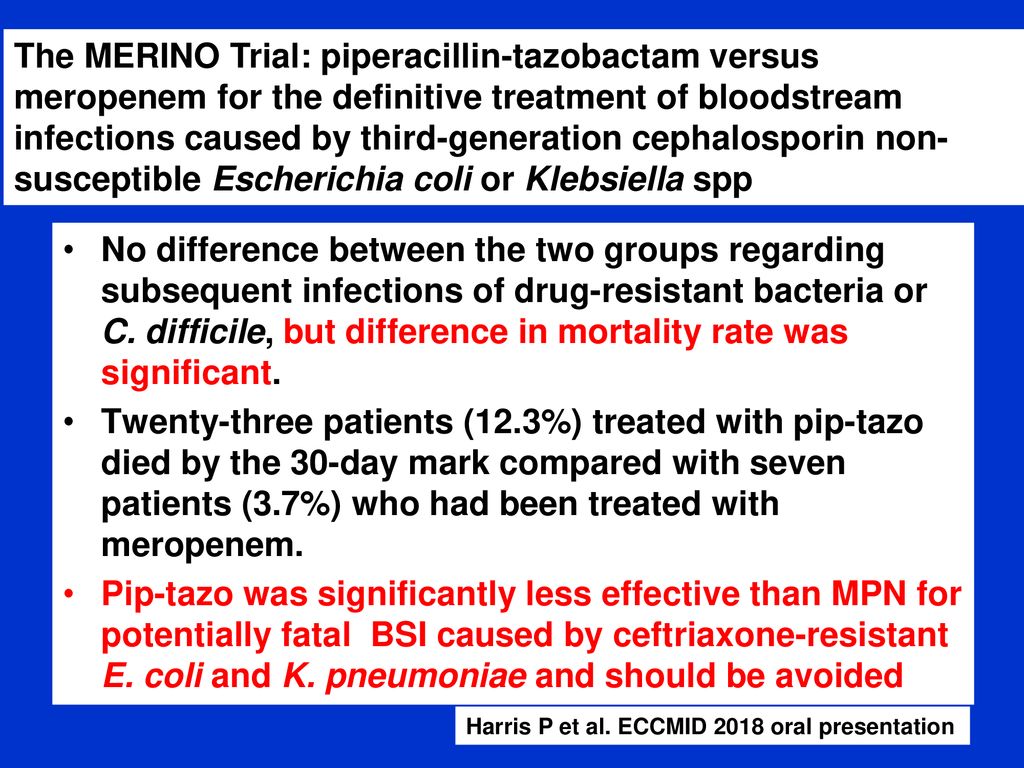
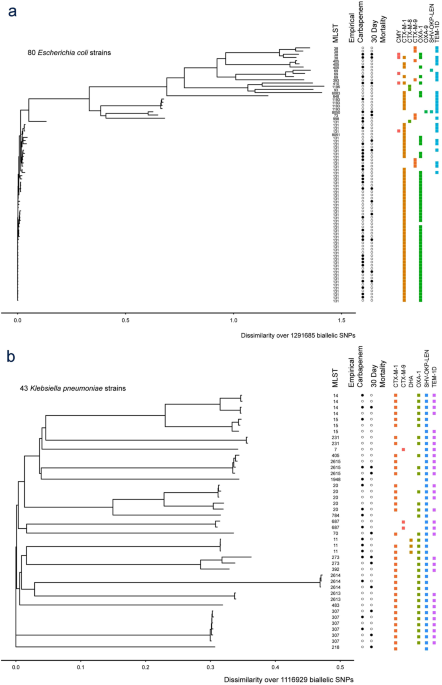
Study protocol for a randomised controlled trial. The merino trial is a landmark study published in september of 2018 that investigated piperacillin tazobactam versus meropenem for the treatment of bloodstream infection caused by ceftriaxone resistant e. 11 this study was an international multicenter open label randomized noninferiority trial of ptz 45 g every 6 hours versus meropenem 1 g every 8 hours for the definitive treatment of e coli or k pneumoniae bloodstream infections resistant to ceftriaxone.
Actrn12613000532707 and actrn12615000403538 and clinicaltrialsgov identifier.