Merino Trial Results
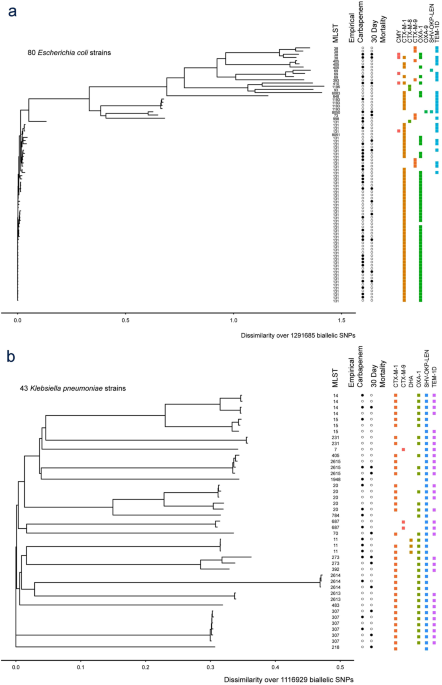
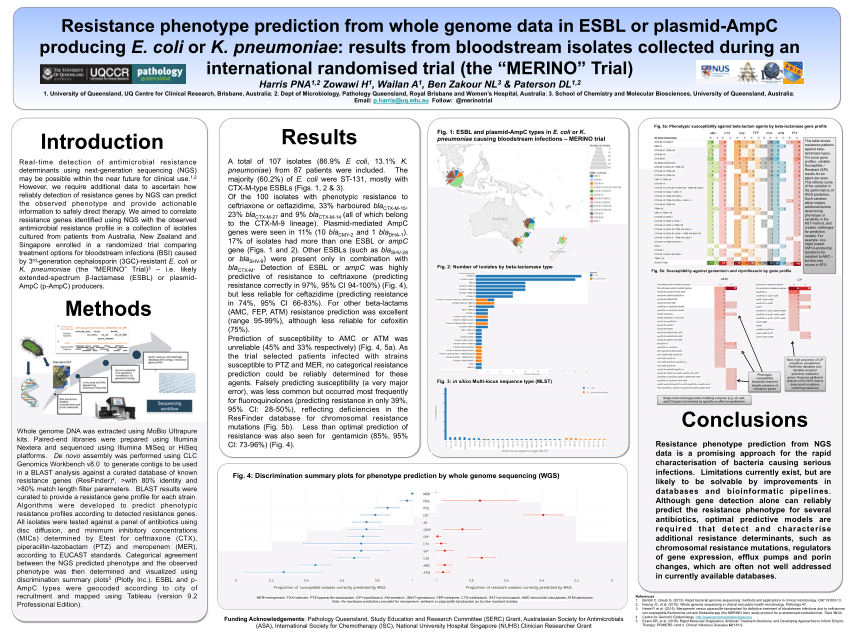
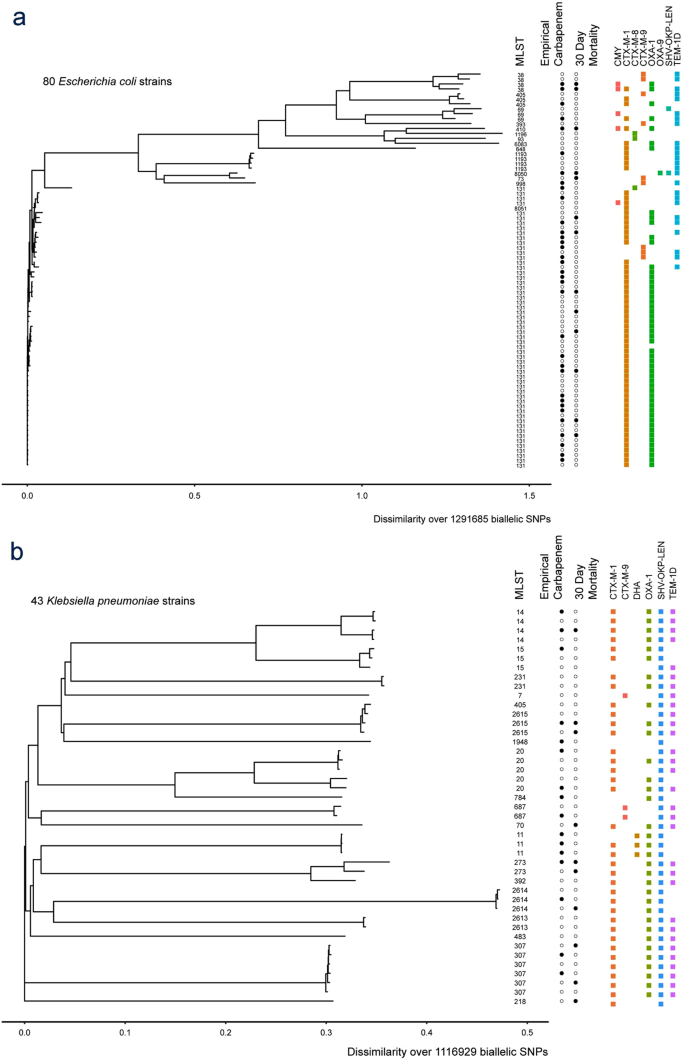
Coli were included of which the majority were st131 614.
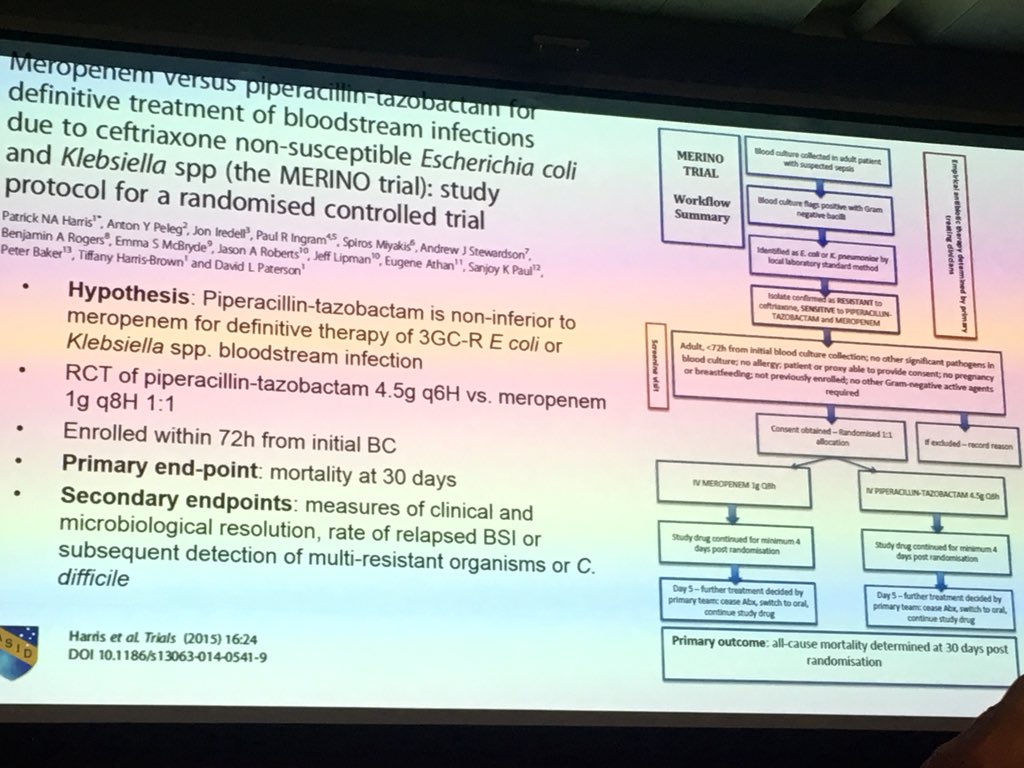
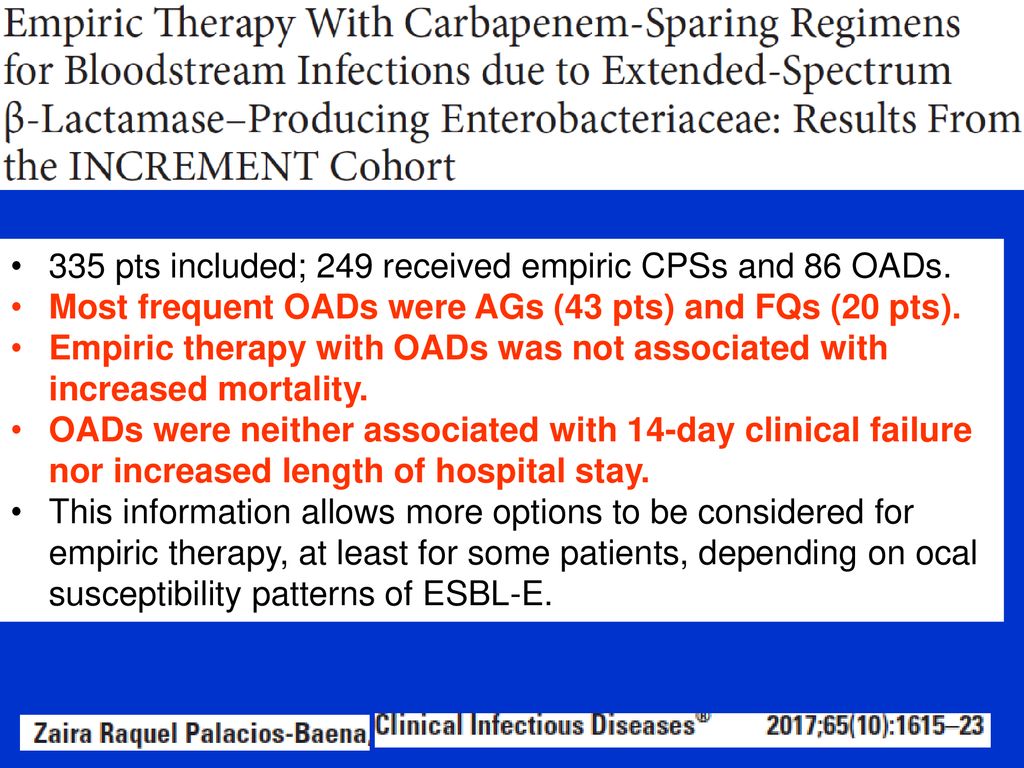
Merino trial results. Study protocol for a randomised controlled trial. Its use is considered controversial given conflicting evidence from 6 studies. The merino trial screened 1646 patients resulting in a total of 379 patients being included in the final analysis.
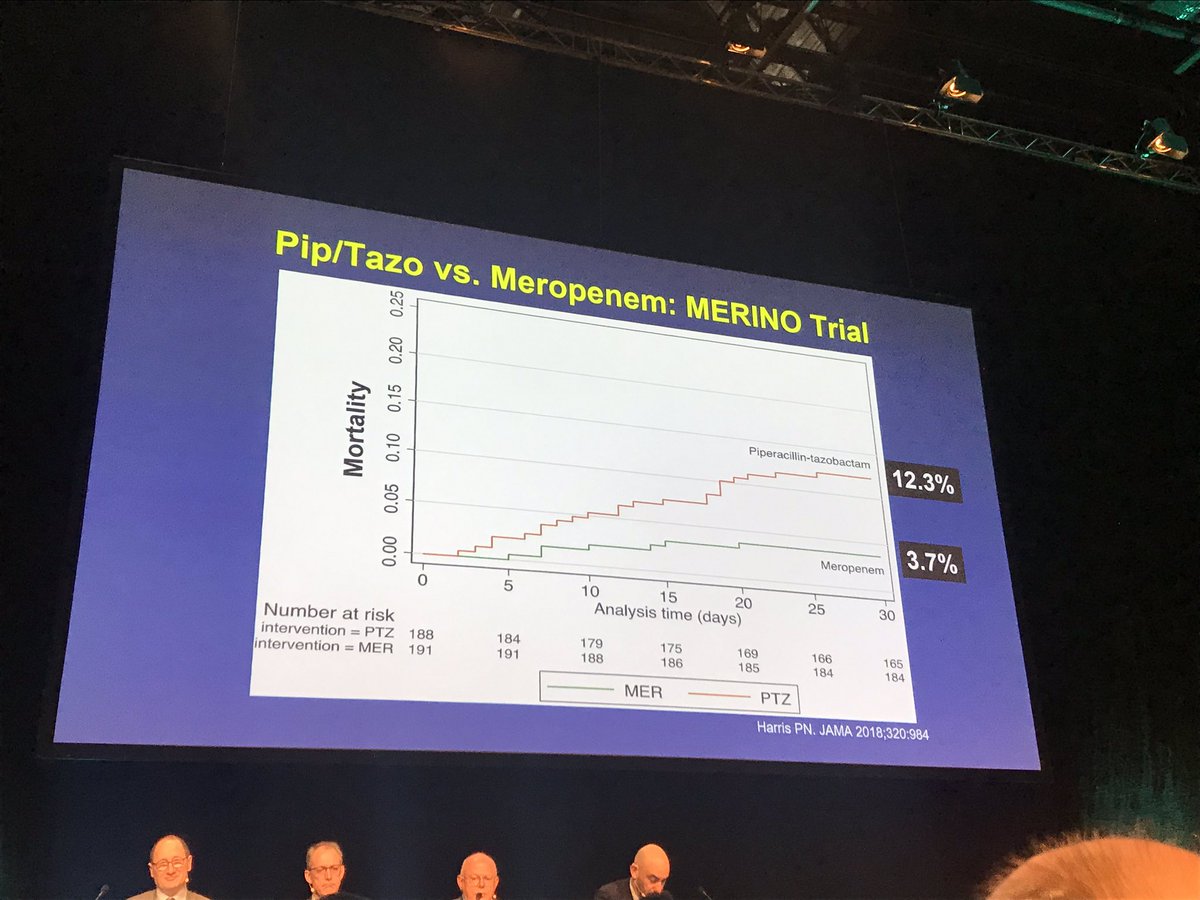
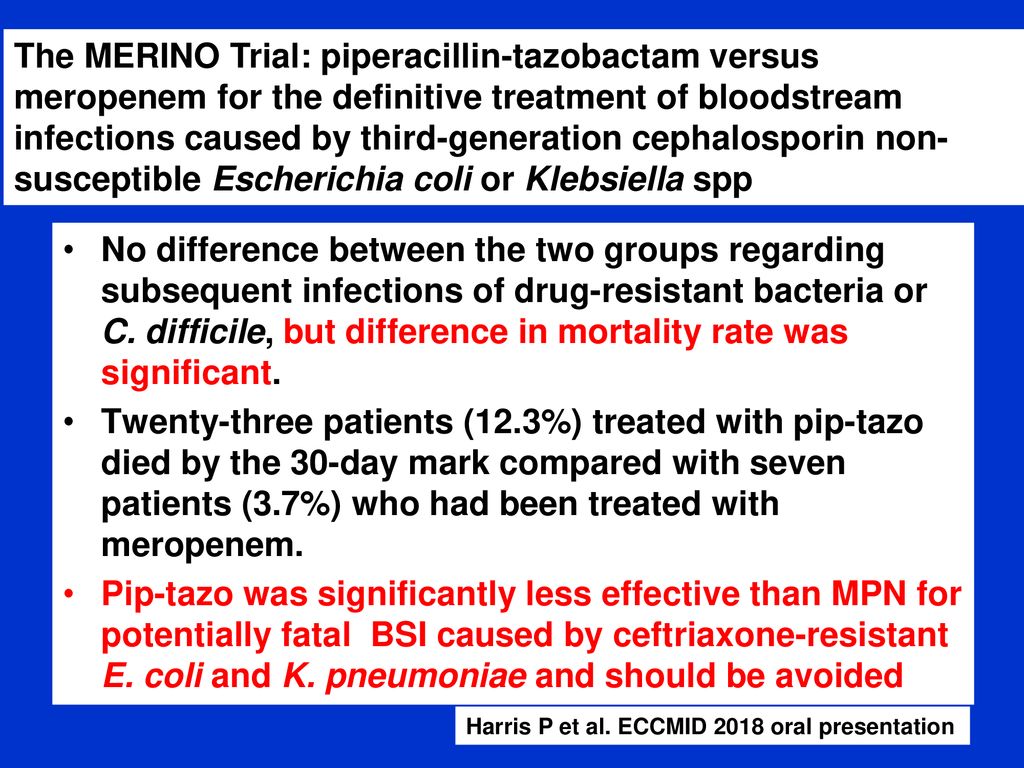
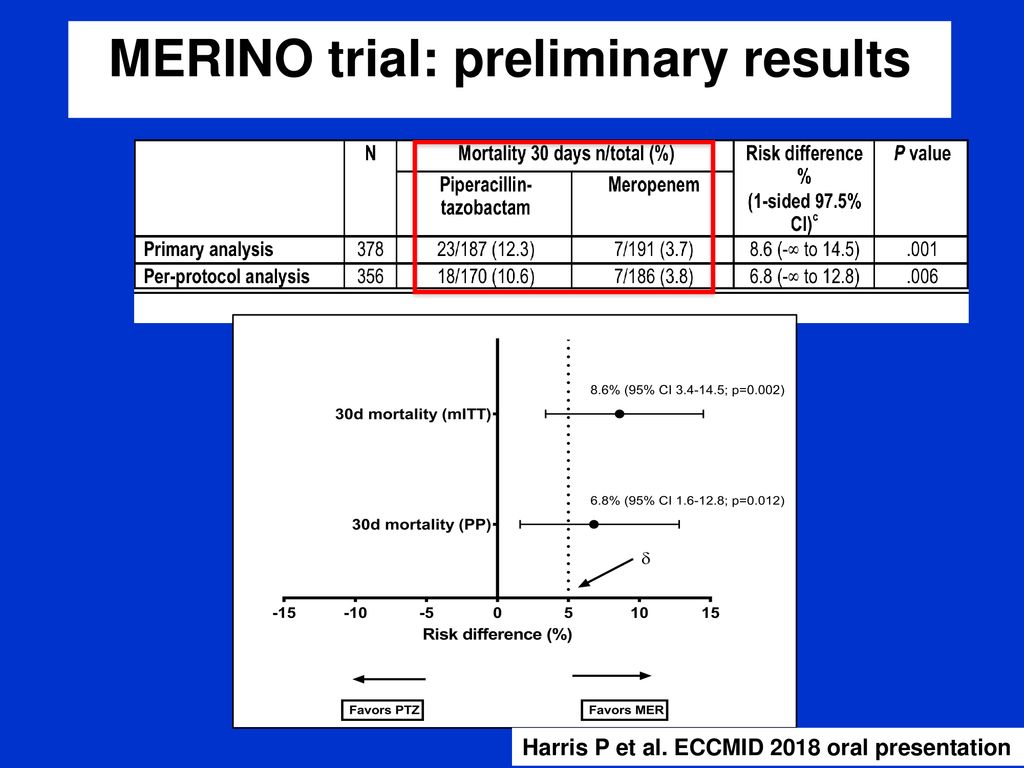
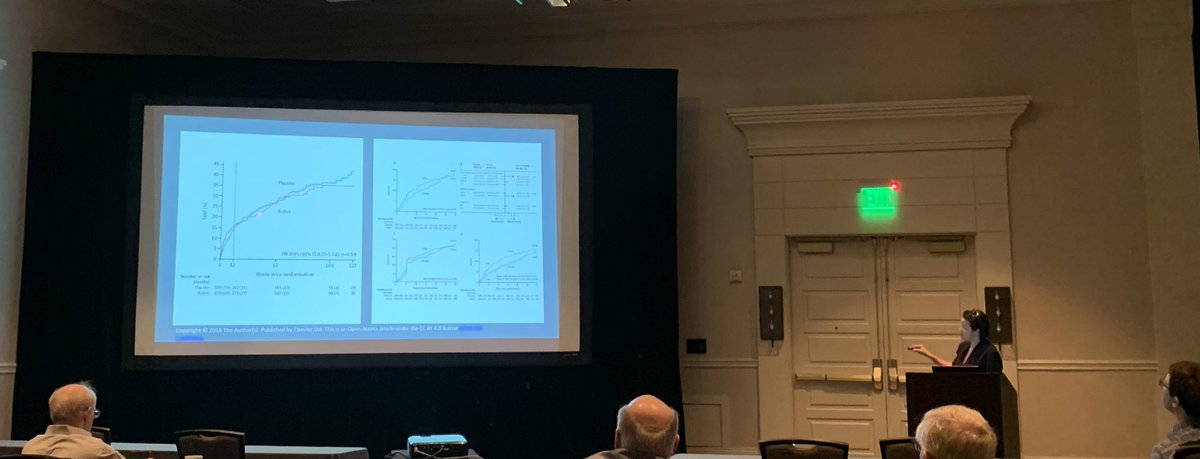
In this interview the lead study investigator provides insights as to their findings and what they have learned through their work. Coli or klebsiella pneumoniae. A total of 23 of 187 patients 123 randomized to piperacillin tazobactam met the primary outcome of mortality at 30 days compared with 7 of 191 37 randomized to meropenem risk difference 86 1 sided 975 ci to.
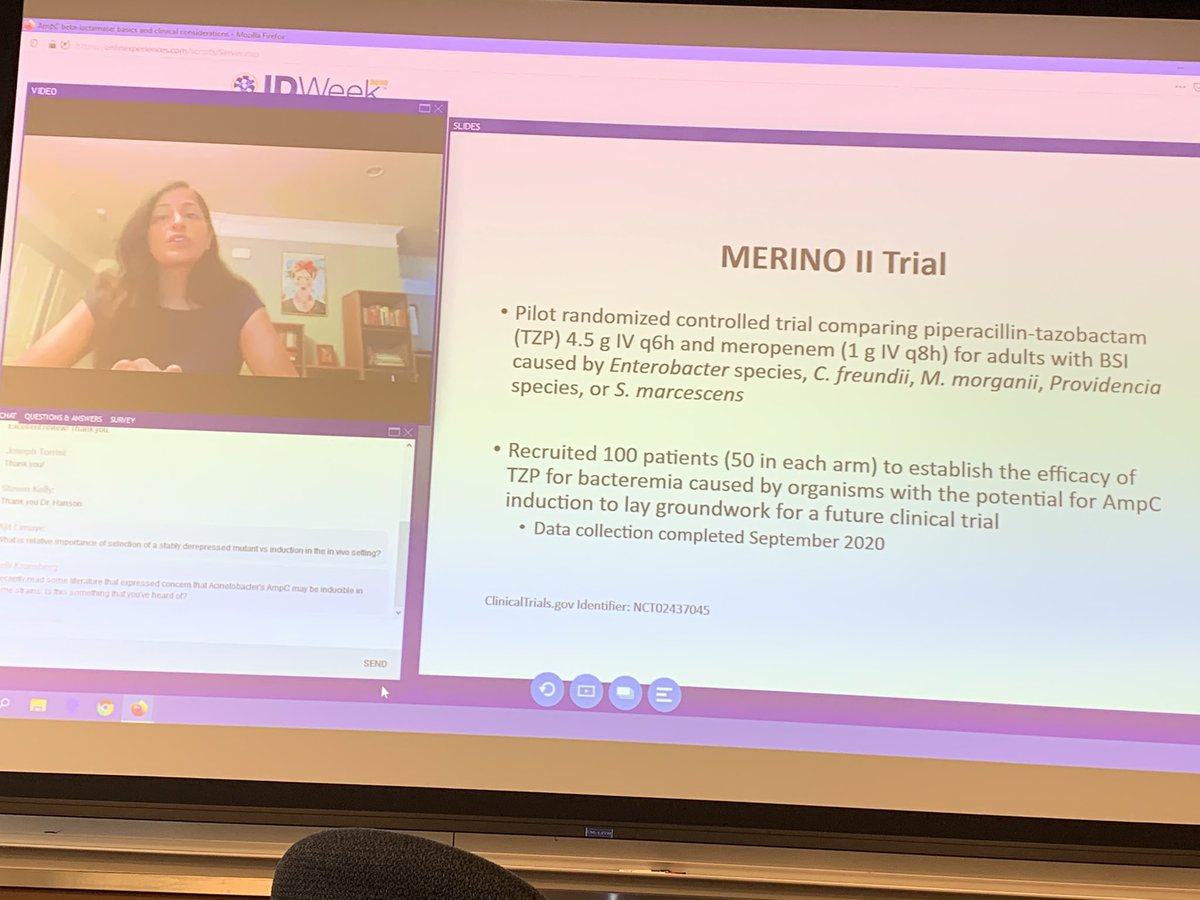
A total of 70 3gc r e. The study was designed to be pragmatic allowing for the treating physician to change medication after a minimum of 4 days and up to 14 days. Trial of meropenem versus piperacillin tazobactam on mortality and clinial response merino ii.
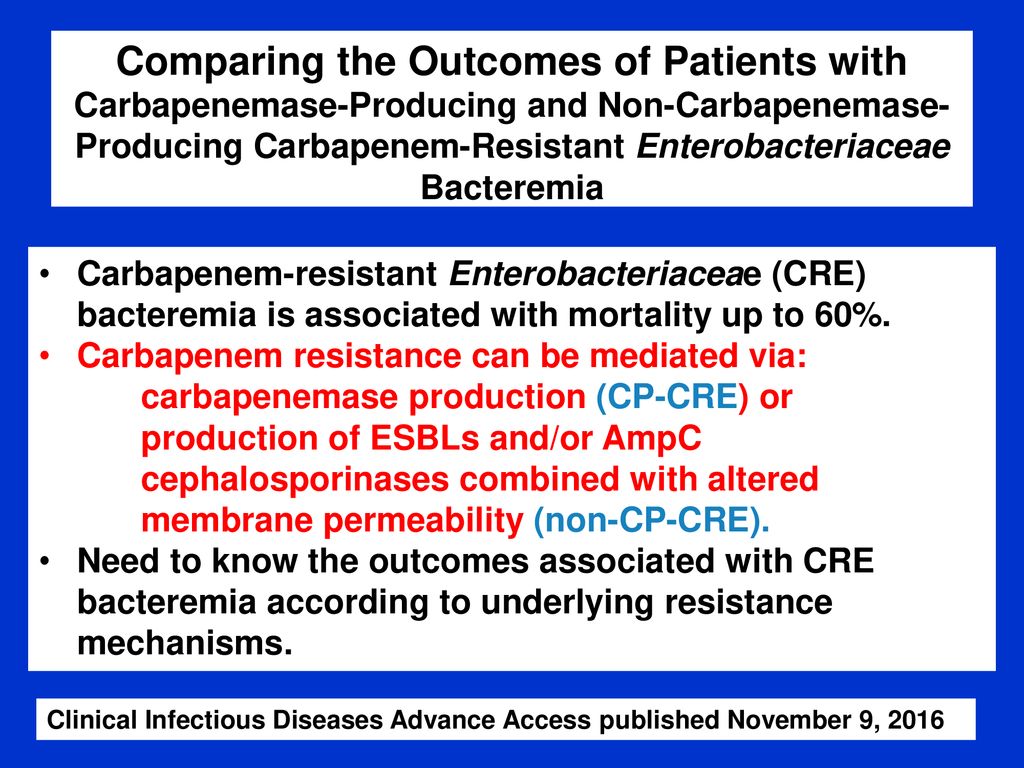
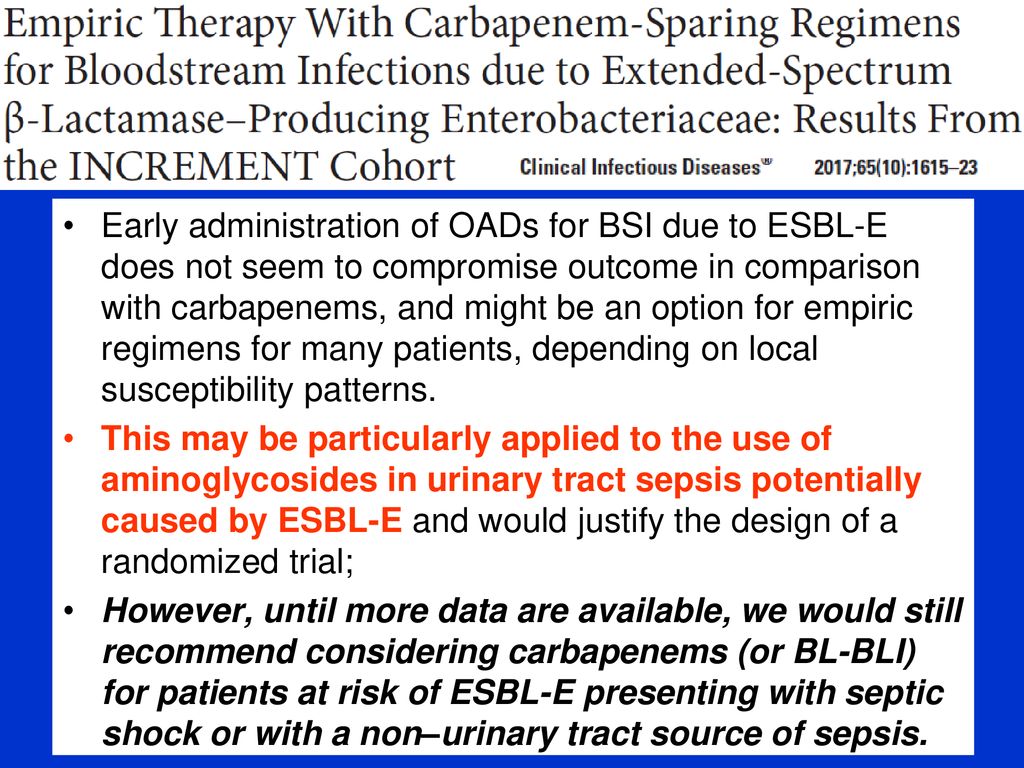
When the infection results from antibiotic resistant bacteria the choice of antibiotic is an extremely important decision. 5 8 their results suggest that for infections with a low inoculum bacteremia because of a binary or urinary source those caused by esbl producing e coli and those in noncritically ill patients ptz offers similar outcomes to a carbapenem based regimen. Some types of bacteria produce enzymes that may inactivate essential antibiotics related to penicillin called beta.
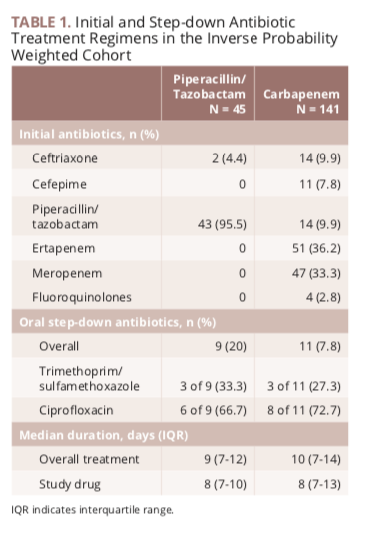
Among 379 patients mean age 665 years. The merino trial is a landmark study published in september of 2018 that investigated piperacillin tazobactam versus meropenem for the treatment of bloodstream infection caused by ceftriaxone resistant e. 478 women who were randomized appropriately received at least 1 dose of study drug and were included in the primary analysis population 378 997 completed the trial and were assessed for the primary outcome.
Findings in this noninferiority randomized clinical trial that included 391 patients with e coli or k pneumoniae bloodstream infection and ceftriaxone resistance the 30 day mortality rate for patients treated with piperacillin tazobactam compared with meropenem was 123 vs 37 respectively. Meropenem versus piperacillin tazobactam for definitive treatment of bloodstream infections due to ceftriaxone non susceptible escherichia coli and klebsiella spp the merino trial. 5 10 four of the studies found no difference in mortality.
Overall baseline characteristics between treatment groups were similar except for an increase in diabetes patients in the meropenem group and an increased number of immune compromised patients in the piperacillin tazobactam group. The merino trial is registered under the australian new zealand clinical trials register anzctr.