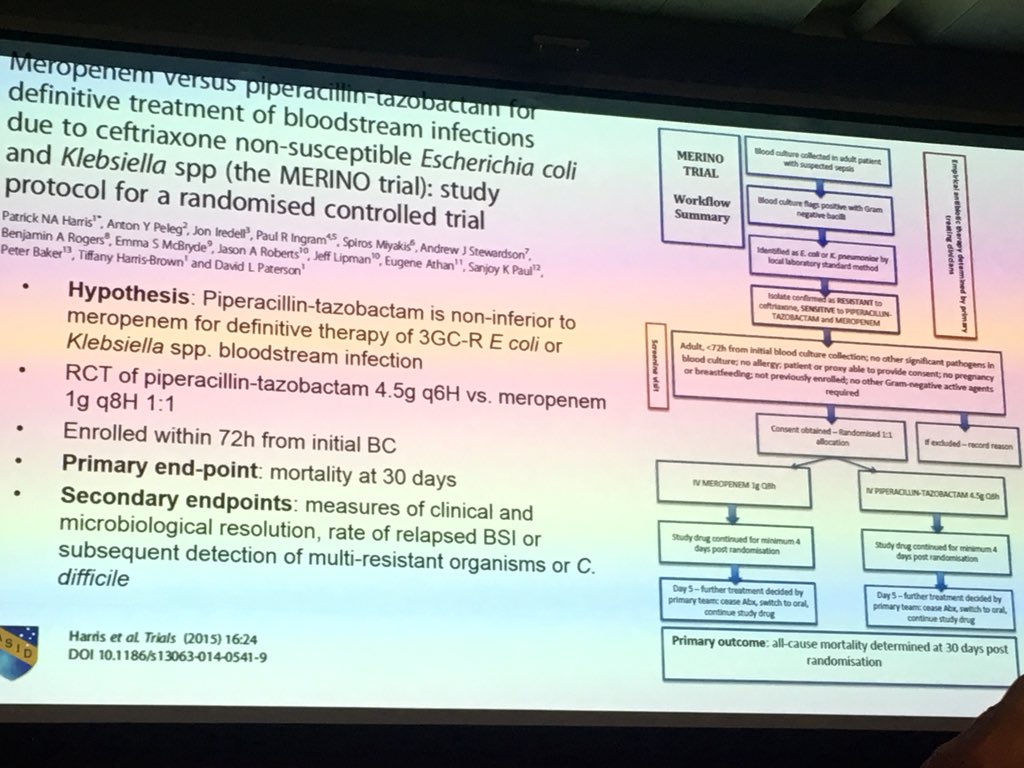
Merino Trial Esbl
1 based on national surveillance data from 2011 the us centers for disease control and prevention estimated that esbl producers accounted for at least 26 000 infections and 1700 deaths annually.
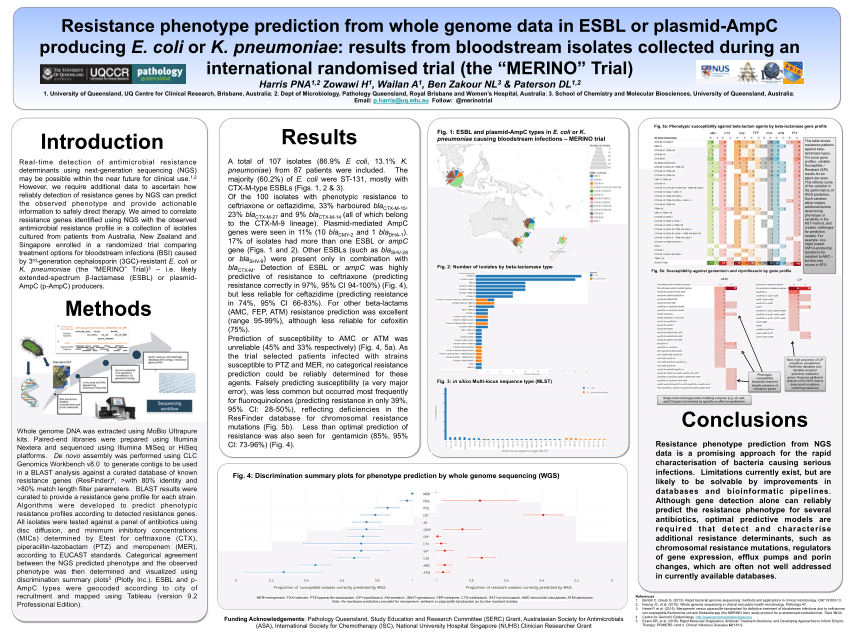
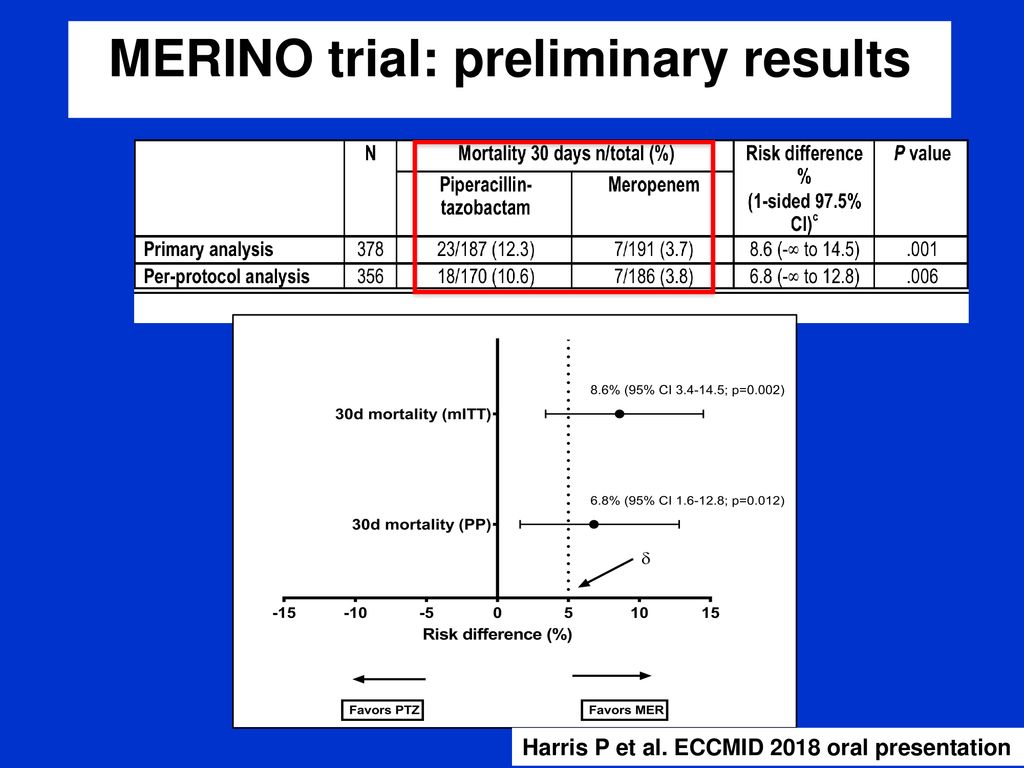
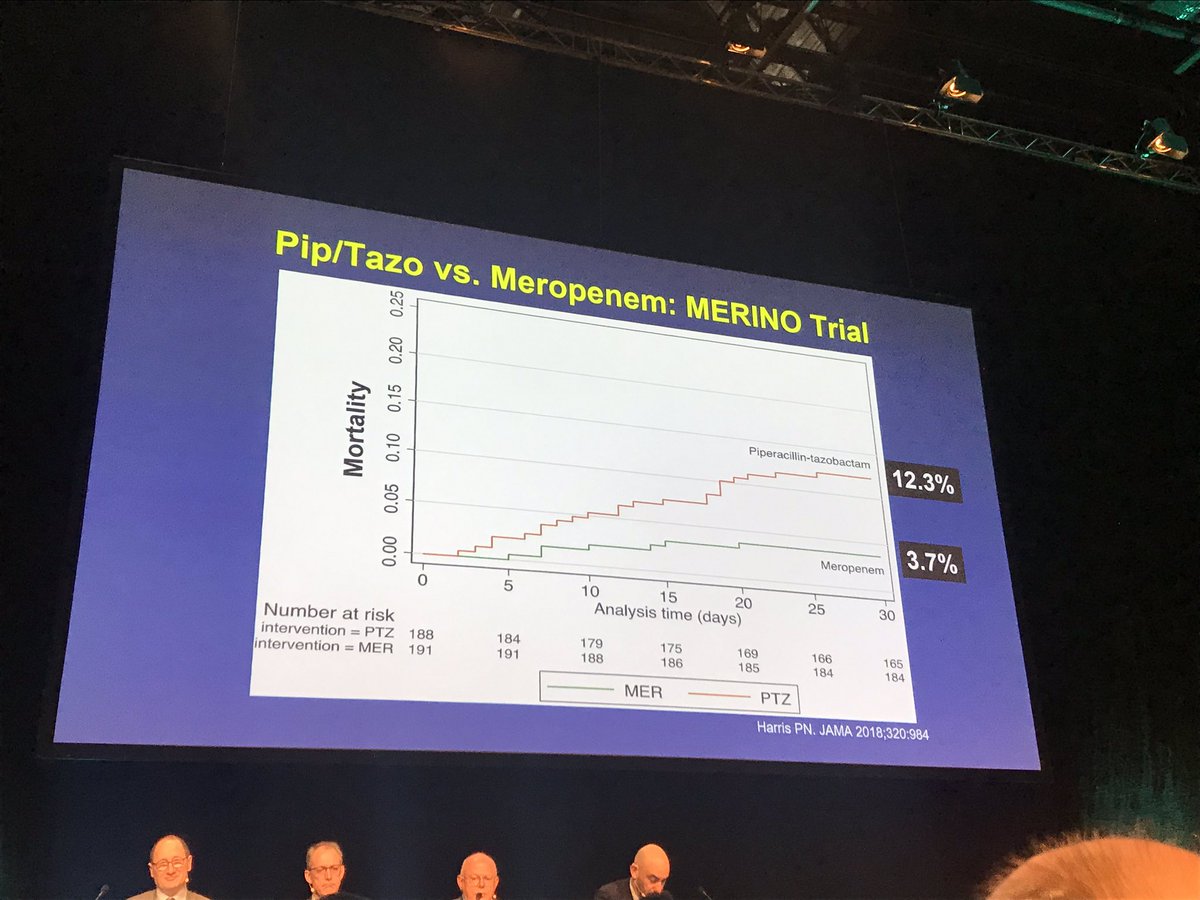
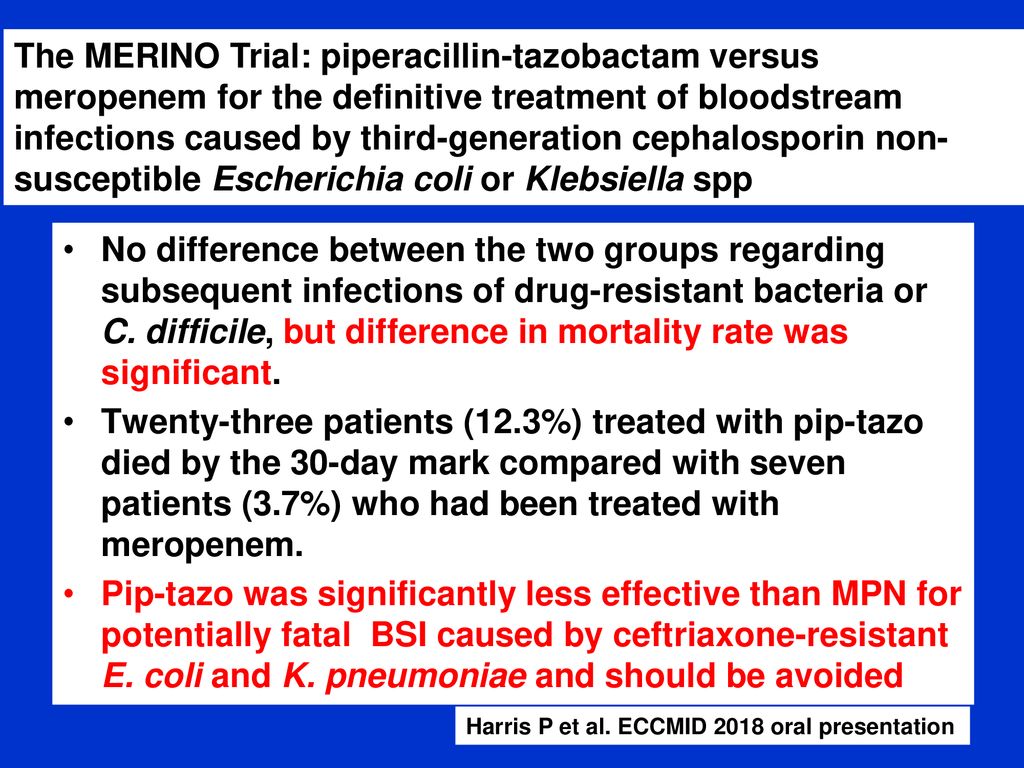
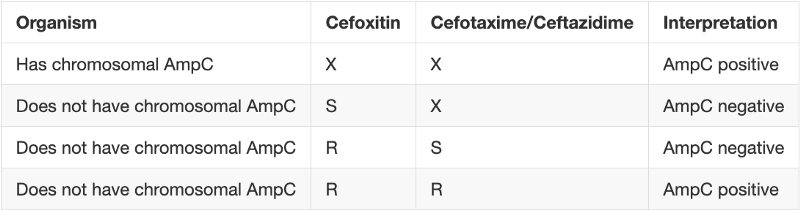
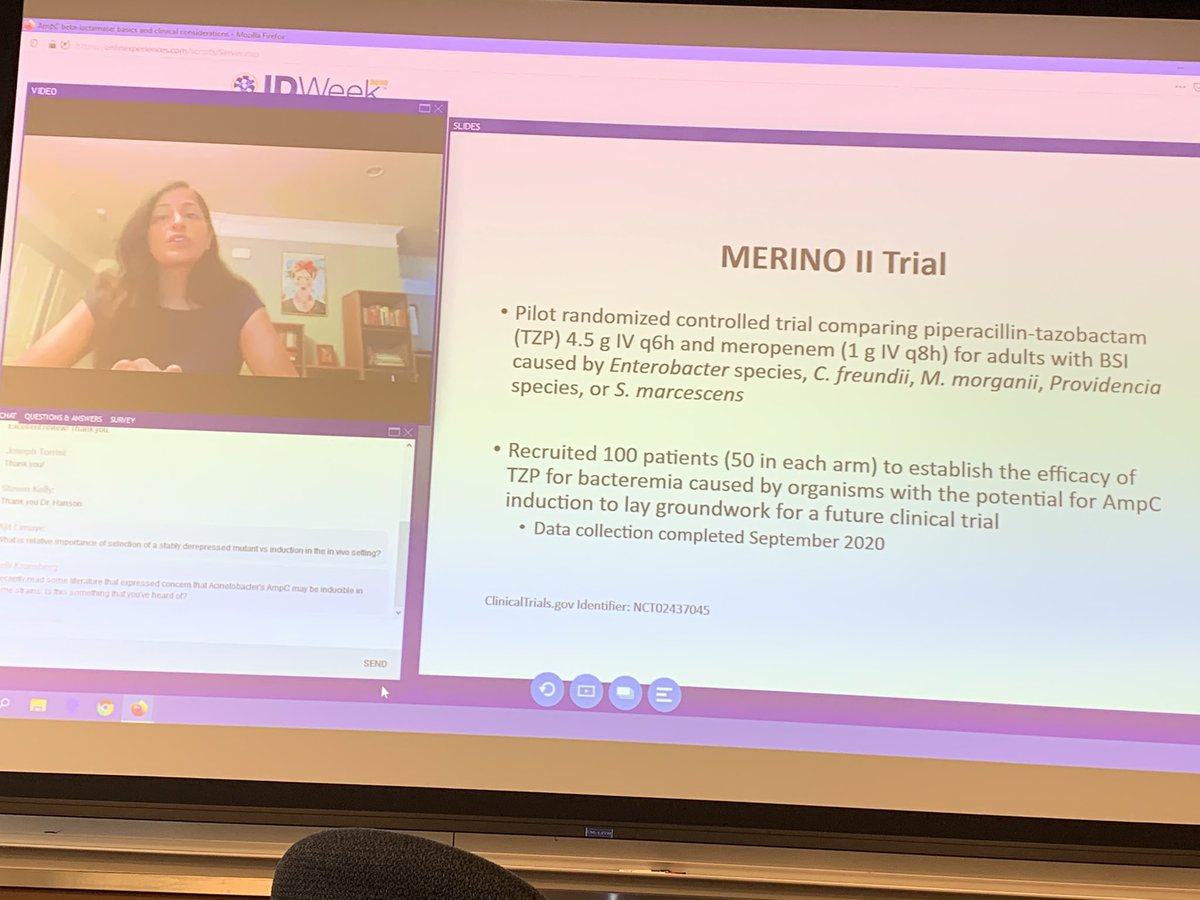
Merino trial esbl. The merino trial and implications for esbl bacteremia treatment. This equivalent time on study and non study drug occurred approximately 83 of the time with only 17 15 meropenem 20 ptz arms of patients on concordant therapy for empiric and study treatment figure 2. The merino trial sought to prove that piperacillintazobactam could serve as a carbapenem sparing strategy for esbl producing bsis and instead found the opposite.
Among 379 patients mean age 665 years. A total of 23 of 187 patients 123 randomized to piperacillin tazobactam. This is a landmark study in helping to define piperacillintazobactams role in treating these infections.
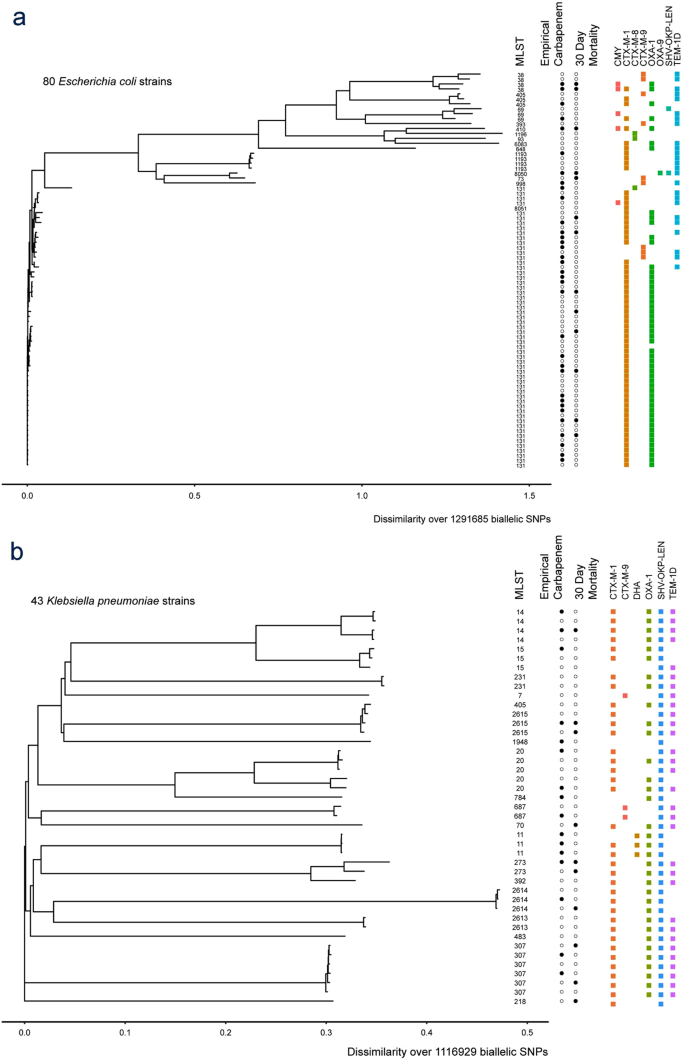
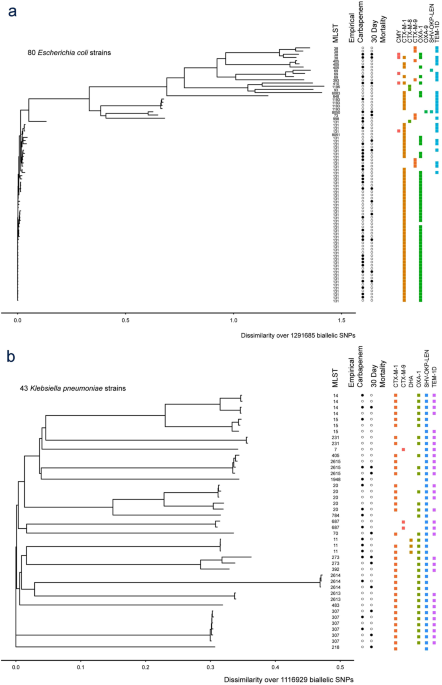
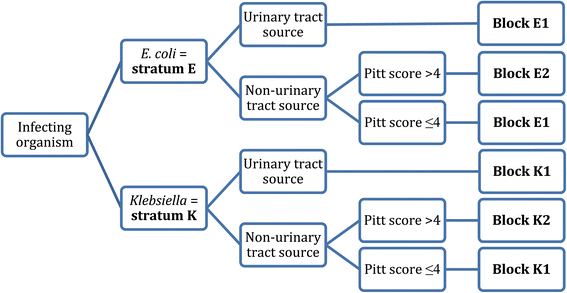
478 women who were randomized appropriately received at least 1 dose of study drug and were included in the primary analysis population 378 997 completed the trial and were assessed for the primary outcome. 11 this study was an international multicenter open label randomized noninferiority trial of ptz 45 g every 6 hours versus meropenem 1 g every 8 hours for the definitive treatment of e coli or k pneumoniae bloodstream infections resistant to ceftriaxone. To address this question the merino trial 3 a multicenter open label trial that randomly assigned patients with escherichia coli or klebsiella spp bacteremia with non susceptibility to ceftriaxone to either standard infusion piperacillin tazobactam 45g6 h or meropenem 1g8 h.
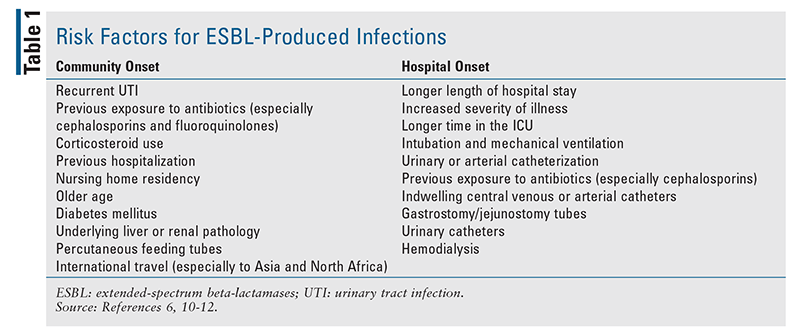
Gram negative bacteria that produce extended spectrum b lactamase esbl enzymes are a global public health concern.